NMR is a sensitive technique and is able to distinguish between nuclei whose
chemical shifts are very similar. The chemical shifts of two nuclei are only equal if the chemical environments of the nuclei are
exactly the same. This only happens if the molecular possesses
symmetry. Consider two isomers of nonanol:
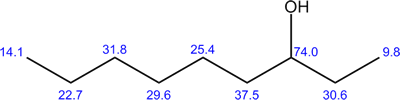
The structure of 3-nonanol is shown to the right. There is no symmetry and all 9 of the carbon atoms are different. The 13C NMR spectrum, shown below, thus consists of 9 peaks. The chemical shifts are shown on the molecule. Notice that the chemical shifts are larger for the 13C nuclei which are close to the electronegative oxygen atom as this deshields the nuclei.
The chemical shifts for the 13C nuclei in the long chain are all different because they differ in their distance from the -OH group and from each other.
|
|
3-nonanol
|
|
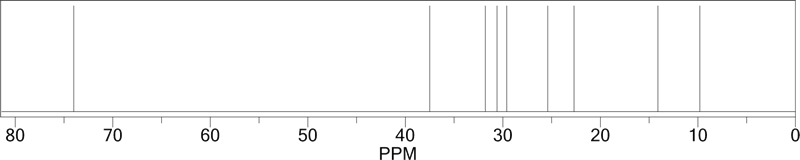
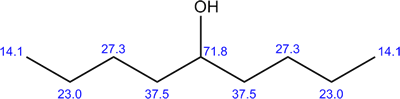
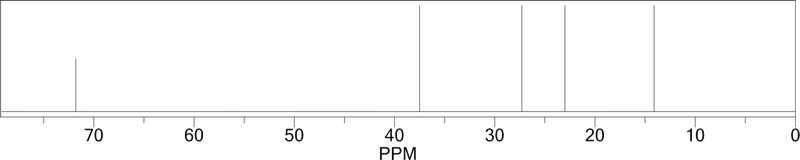
| The structure of 5-nonanol is shown to the right. In this case, every carbon on the left of the OH has an equivalent on the right of the OH. There is a mirror plane running through the central C-OH group. Due to this symmetry, there are only 5 different types of carbon atoms. The 13C NMR spectrum, shown below, thus consists of only 5 peaks. The chemical shifts are shown on the molecule..
|
|
5-nonanol
|
|
The first stage in working out what the NMR spectrum of a molecule will look like is to identify have many different environments there are in the molecule.
Look for symmetry!